|
|
1.IntroductionInfrared neural stimulation (INS) is a stimulation modality that employs low-energy, pulsed infrared light to reliably excite nerves.1 The resulting action potentials in peripheral nerves are produced without direct contact from the stimulating optical fiber probe.1–3 We have demonstrated the selectivity of INS to activate individual nerve fascicles, and the use of laser energy for neural stimulation allows artifact-free electrical recordings to be obtained close to the site of stimulation in animal models ranging from Aplysia to nonhuman primates.4–7 These features make INS an attractive alternative to clinical electrical neurostimulation, especially in certain clinical applications where a high degree of selectivity is required. Here, we report the first successful application of INS for activating human nerves in vivo. The advantages of INS provide the ability to circumvent certain limitations of clinical electrical stimulation (ES) used for diagnostic and therapeutic applications. The ability to stimulate surface area of neural tissue (e.g., in peripheral nerve reconstruction surgery or facial nerve monitoring during vestibular schwannoma resection) usually requires an electrode to physically contact the surface or impale neural tissue, resulting in local damage to neural tissue. Yet the area encompassed by the stimulation probe is not seen with ES, and current spread beyond the stimulation point of contact concerns surgeons wishing to monitor the continuity of neural structures immediately under the stimulation probe. Since ES usually produces a Gaussian distribution of the stimulation field beyond the probe’s contact with the tissue, it is not ideal when trying to confine the point of stimulation to a visual target, leading to stimulation of distant neural structures and potential misdiagnosis of whether the local connectivity of neural structures is viable. Additionally, current spread associated with ES can excite multiple neural structures near the electrode leading to unwanted stimulation of adjacent neural structures and can limit the precision in therapeutic applications, such as deep brain stimulation of subthalamic nucleus or cochlear implants.8,9 Conversely, with INS, the area encompassed by the stimulation field follows a near-rectangular drop-off when the INS beam is properly collimated in the probe. This results in a visible point of stimulation in the tissue that corresponds to an area only covered by the INS beam and not any further. INS, therefore, is a method of stimulation with a more predictable spatial stimulation field than ES. INS also allows for more resolution when recording the electrical response of neural structures to stimuli. Electrical recording of the neural response becomes more challenging as the distance between the stimulation and recording electrodes decreases because the electrical artifact can overwhelm evoked neural signals, limiting the ability of the clinician to assess the efficacy of applied stimulation.10–12 For instance, under most normal clinical scenarios where cranial nerve monitoring is desired, it is technically very difficult to record facial electromyogram (EMG) signals and nearly impossible to record electroneurogram signals when the facial nerve is stimulated anywhere along its pathway. This is due to the presence of the stimulation artifact seen within the recordings, and it occurs, in part, because the stimulation and recording methods occur in the same modality. However, use of laser energy for stimulation to detect an electrical response in the nerve or muscle can occur virtually side-by-side, since the irradiated field by the laser does not produce an electrical signal in-and-of-itself in the recording electrode. This would allow placement of recording electrodes, for instance, directly on the facial nerve in close proximity to the point of stimulation using INS technology. The limitations of clinical neurostimulation are most pronounced in peripheral nerve neural monitoring applications, where there is a diagnostic need for precise physiological identification and stimulation of peripheral and cranial nerves. The present use of electrical microstimulation probes is seen with numerous surgical procedures involved in either safely identifying portions of a peripheral nerve to avoid damage or to select such fibers for therapeutic treatment; however, clinical ES has certain limitations that can lead to misdiagnosis of neural tissue. Examples of the value of such selective precision include the routine use of facial nerve stimulation during resection of acoustic neuromas, where fascicles of the facial nerve may be quite splayed over the back surface of the tumor. The use of small electrical probes in identifying the facial nerve may give a false sense of spatial precision when the stimulation energy is excessive and activates neural fibers that are quite distant from the operative site.13,14 Another surgical example is the use of electrical microstimulation for identification of viable peripheral nerve fibers during neural reconstructive procedures. In these procedures, peripheral nerve fascicles need to be identified that are electrically viable versus nonviable and, therefore, requiring a nerve graft. Grafted sections of the nerve are usually 1 to 2 mm in diameter and represent only a portion of the peripheral nerve needing an anastomotic graft usually harvested from elsewhere in the patient. It is, therefore, valuable to identify with precision the appropriate sections requiring resection and anastomosis within a larger peripheral nerve undergoing repair. In both of these examples, an alternative neurostimulation modality is needed that spatially confines the region of stimulation. INS is a viable alternative neurostimulation modality ideally suited to provide the needed spatial precision in diagnostic neurostimulation applications of peripheral and cranial nerves. The selectivity and delivery of INS has been well characterized by our group in past experiments in the rat sciatic nerve.3–5 We have demonstrated the efficacy and safety of INS for stimulation of peripheral nerves, where a safety ratio exists between damaging radiant exposures and threshold energy levels needed for stimulation.3 The initial results from our group’s efforts have led to the development of INS for clinically relevant diagnostic and therapeutic applications, including neural monitoring,5,15–18 cochlear and vestibular stimulation,19–22 cardiac pacing,23–25 and applications in the central nervous system.7,26–30 Each of these studies demonstrates that INS is capable of producing high levels of spatial precision in stimulating excitable tissue. The high spatial precision associated with INS results from the biophysical mechanisms that leads to the excitation of neural tissue. The underlying biophysical mechanism of INS has been known to activate neural tissue through a transient thermal gradient.4 Wells et al. demonstrate that the thermal gradient is generated from absorption of infrared light by tissue water and that the thermal energy is spatially confined to the irradiated volume of tissue. The volume irradiated by infrared light is determined from the spot size of the laser beam and the penetration depth of the light into tissue. These characteristics of the thermal gradient give rise to the high spatial precision seen with INS and have been confirmed by others modeling the biophysics associated with the INS thermal gradient.4,31 Recent studies have demonstrated how the thermal gradient is transduced into neural signals. Shapiro and colleagues provided evidence that the INS-evoked thermal gradient depolarizes lipid membrane bilayers through a thermally mediated change in membrane capacitance independent from specific ion channels. This simplified mechanism indicates that all neural tissues can be excited using INS. Alternatively, the authors hypothesized that other cellular mechanisms may be involved.32 A separate study confirmed this hypothesis by demonstrating that heat-sensitive TRPV4 channels were the primary mechanism behind INS-evoked action potentials in retina ganglion cells.33 These mechanistic findings support INS as a robust stimulation method that can be used as an alternative clinical stimulation method to augment current diagnostic and therapeutic neurostimulation applications; however, its efficacy and safety in human surgery must be first validated before clinical application of INS is realized. The goal of this study is to demonstrate the safety and efficacy of this technology in humans during a clinical procedure that requires ES for use as a diagnostic tool during neurosurgical procedures that require detailed nerve root mapping. In order to accomplish these goals, we stimulated dorsal spinal roots identified for transection in patients undergoing selective dorsal rhizotomy for the treatment of medically refractory spasticity typically seen in patients with cerebral palsy. Selective dorsal rhizotomies (SDR) were chosen for several reasons: (1) the human dorsal root is of similar size as the rat sciatic nerve, (2) ES and EMG recordings are routinely used to precisely identify specific dorsal roots, (3) and it is a procedure whereby a nerve (dorsal spinal root) is intentionally transected, thereby allowing the option to harvest a small (1 cm) section of the nerve without added deficits. These characteristics of the SDR procedure allowed for direct assessment of INS for efficacy and safety in human nerves and validate our results in rat sciatic nerves through EMG recordings and histological analysis.3,5 We report that INS effectively stimulates human dorsal roots with the same high spatial precision demonstrated in animal models without generating a stimulation artifact on recording electrodes. 2.Materials and Methods2.1.Patient RecruitmentAll protocols and procedures implemented during this preclinical trial were approved by the Vanderbilt University institutional review board (IRB# 050822, NCT00575536). Informed consent was obtained in all cases. Seven patients, ages 3 to 16 (, ), undergoing SDR for the treatment of lower extremity spasticity were recruited for this study from 2006 to 2010.34,35 Inclusion into this study required patients to undergo electrodiagnostic EMG monitoring as a normal part of the procedure, and it allowed for direct comparison of the physiological response between INS and ES. Patients were excluded if a sufficient segment of nerve () could not be identified for resection at the time of surgery as determined by the pediatric neurosurgeon or if the patient was medically unstable to tolerate an additional 20 min of experimental testing during the surgery. 2.2.Surgical ProcedureSDR is a standard surgical procedure used to identify dorsal afferent spinal roots that contribute to excessive muscular tone or spasticity in the lower extremities.36 Once identified, a select number of nerve roots are cut while others are spared for each lumbar segment, leading to an overall decrease in the afferent sensory tone of that region of the spinal cord and presumably diminished spasticity. The surgery normally involves performing a laminectomy of the lumbar spine, opening the dura overlying the lumbar spinal nerve roots, and physiologically identifying the dorsal spinal nerve roots involved in the spastic reflex using standard electrical stimulation techniques. Note that in the normal response to threshold stimulation of a dorsal rootlet, the afferent signal is transmitted through a spinal cord synapse to alpha motor neurons usually located within the myotome, which, in turn, elicits a muscle response monitored by EMG recordings. During the surgical procedure, however, abnormal responses are seen well beyond the myotome involved in the segmental reflex arc and can be recorded in simultaneous EMG recordings in flexor and extensor muscle groups of both legs. Once a dorsal rootlet is identified as a significant contributor to EMG responses in a large number of muscle groups, a certain percentage of the abnormal nerves are sectioned. Electrical stimulation parameters to elicit threshold responses are recorded in response to single pulse electrical stimulation (1 to 10 mA, pulse duration) of individual spinal nerves (L2—S1). Physiological abnormal spinal roots involved in spastic reflexes are identified by stimulating the rootlet with a train of threshold stimulatory electrical pulses delivered at 20 Hz. Nerve roots exhibiting spastic firing patterns or incorrect wiring to multiple muscles as determined from surgical monitoring of EMG signals are identified for sectioning.37 2.3.Laser Setup and INS ParametersOnce a nerve has been designated for sectioning, INS was performed using a clinical holmium:YAG laser () to stimulate two to three locations on the nerve for 10 s at 2 Hz. The clinical laser operates at higher radiant energies and repetition rates than the stimulation parameters required for this study. A light tight optical box was constructed to control the output radiant exposure and to reduce the repetition rate to 2 Hz (Fig. 1). Since animal and human nerves have similar optical properties, we expected the radiant exposure required for both stimulation and damage in animal nerves to be comparable in human peripheral nerves: 0.34 to and 0.7 to , respectively. Light output from the clinical laser (, 6 Hz) was modified to an adjustable output energy from 0 to 25 mJ (0 to ) at 2 Hz delivered through a handheld optical fiber probe held by the surgeon. Fig. 1Schematic diagram of optical box for modifying high-power and high-frequency clinical system to optimal parameters for infrared neural stimulation (INS). High-energy laser light entered the box from a optical fiber [multimode fiber (FG550LEC), Thor Labs, ] connected to the clinical system. A biconvex lens (, Thor Labs) was used to focus the divergent beam from the input fiber through a beam splitter and couple the attenuated beam of laser light into the delivery fiber (). A photodiode, placed between the input fiber and lens, detected high-frequency input light pulses and triggered an optical shutter to adjust the output frequency to 2 Hz using a pulse generator. An external micromanipulator attached to the optical fiber mount inside the black box allowed for fine control of the light coupling efficiency and, thus, the amount of light entering the output core optical fiber (). The output end of this fiber was mounted onto a sterilized, handheld optical fiber probe (, ) for ease of use by the neurosurgeon during stimulation. A red HeNe aiming beam output from the clinical system was maintained through the probe output, providing a known stimulation site. The laser-probe system was footswitch controlled. 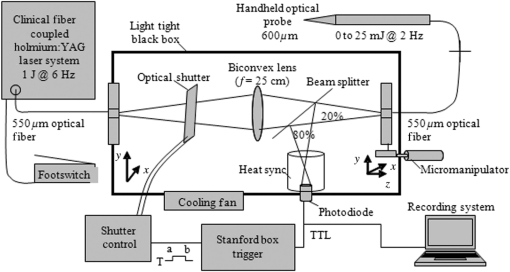 The first five spinal nerves selected for resection were stimulated using infrared energy before the nerves were resected. The neurosurgeon maintained the distance between the nerve and handheld optical probe tip between 1 and 2 mm during stimulation, yielding a spot size of , estimated using the angle of divergence of light out of the fiber. Stimulated nerves were surrounded by a sterile silastic sheet to insure no aberrant laser exposure of adjacent normal tissue and help guarantee that recorded muscle responses were elicited due to the laser energy incident on the chosen nerve root. Each nerve was irradiated on two to three adjacent sites before marking, sectioning, and harvesting of the nerve. One or two sites on each nerve was irradiated with 20 laser pulses (2 Hz) with a constant radiant exposure between stimulation and damage threshold as defined in mammalian studies (0.3 to ). One to two millimeters adjacent to this spot, the nerve was stimulated with 20 pulses (2 Hz) using laser energies greater than the damage threshold but less than the ablation threshold identified in mammalian studies (control lesion, 2.0 to ).3 The nerve segment, measuring , was harvested and submitted for histological analysis. 2.4.Data Recording and AnalysisThe evoked EMG data were collected in a standard clinical electrophysiological setting and protocol. EMG responses to optically and electrically stimulated nerves were recorded with the clinical electrophysiological system used routinely in the surgery (Nicolet Endeavor; Nicolet Biomedical Inc. Madison, Wisconsin). Muscle-EMG recordings were simultaneously monitored and collected from all major muscles groups of both legs, namely, the adductor longus, vastus lateralis, biceps femoris, medial gastronemeus, peroneus longus, and anterior tibialis muscles in response to ES and INS. Once a rootlet was identified for sectioning, a 10 s continuous EMG recording was captured during INS beginning 2 ms prior to stimulation of the nerve through screen capture every 2 s during INS. Each response was recorded after the signal was amplified () and bandpass filtered (50 to 500 Hz). A comparison was made with electrical and INS performed at the same or adjacent points on the nerve and the resulting muscle action potentials recorded. Successful stimulation of the rootlet by INS was determined by the presence of EMG signal in muscle groups activated by the diagnostic ES stimulation (Fig. 2). Muscle groups exhibiting spastic activity (indicated by high-frequency EMG activity) during the entirety of the procedure were excluded from analysis in determining INS activation. Fig. 2Pulsed infrared light evokes compound muscle action potentials through stimulation of human dorsal root. Electromyogram (EMG) recordings from 12 muscles in the lower extremities (top six traces = left leg, bottom six traces = right leg) in response to electrical stimulation (20 Hz, , 1 s) and INS (2 Hz, , 10 s) on a left dorsal root L4. (a) Responses obtained from 20 Hz train of electrical stimulation () visualized on a voltage scale of . EMG recordings indicate a response in all left and right side muscles. (b) Reponses obtained from INS () of same nerve with a voltage scale of . A single response is observed in the left adductor muscle (denoted by black arrows). 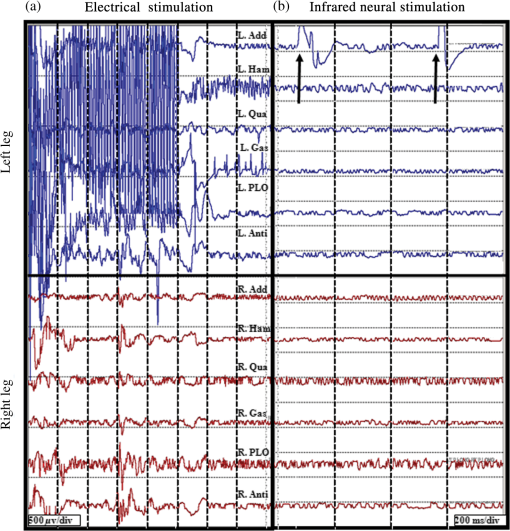 2.5.Tissue Preparation and AnalysisOnce a nerve that was identified for sectioning was exposed to INS, the nerve stimulation sites were marked with methyl blue ink to aid in histological analysis. The nerve segments containing the two to three irradiated zones were excised (1 to 2 cm), immediately placed in formalin, and prepared into slides of thin longitudinal sections cuts perpendicular to the stimulation sites. Sections were sent for an independent blinded review of acute histological changes occurring from laser stimulation, interpreted by an expert in histopathology associated with laser-tissue irradiation (S.T.).38 Areas of coagulation, axonal disruption, and perineurium damage were assessed using light microscopy and routine hematoxylin and eosin staining. Changes sought in laser irradiated tissue follow the methods outlined for acute studies in previous mammalian studies.3 These criteria help define the following three-point grading scheme assigned to each specimen indicating the extent of damage at the site of optical stimulation: 0, no visible thermal changes; 1, pathological changes in the section that cannot be confirmed as thermal damage (mechanical or thermal), 2, presence of a thermal lesion within the tissue section. 2.6.Statistical AnalysisIn order to determine the probability of damage associated with INS radiant exposures, the binary data (damage versus no damage) resulting from histological analysis were calculated by fitting the data to a probit model typically used to determine damage thresholds associated with laser irradiation.3 Probit analysis allows for the binary damage data to be fit to a continuous distribution function (CDF) to quantify the probability of damage at a given radiant exposure. The binary data were input into Probit v2.1.2 (Litton TASC, San Antonio, Texas) to obtain the CDF that describes the probability of damage as a function of radiant exposure.39 The resultant CDF curve was used to help determine a range of nondamaging radiant exposures for stimulation of human tissue. 3.Results3.1.Infrared Neural Stimulation Evokes Neural Activity in HumansIn the seven patients studied, INS successfully activated individual muscle groups in dorsal rootlets first identified with ES. Figure 2 compares the EMG recordings of ES to INS of a dorsal root identified for sectioning. EMG recordings were taken from the following six muscles in both the right and left leg (12 total EMG recordings): adductor longus (R&L ADD), biceps femoris (R&L HAM), vastus lateralis (R&L QUA), medial gastronemeus (R&L GAS), peroneus longus (R&L PLO), and anterior tibialis (R&L ANTI). Note that the threshold ES (20 Hz, 2 mA train lasting 0.5 s) of a single dorsal nerve root branch elicited EMG responses in over 10 muscles, both ipsi- and contralateral limbs. In this particular patient, this nerve root was considered to be pathologically involved in the spasticity of the patient and was selected for transection. Once identified as a root that was to be transected, INS was performed using a handheld optical fiber probe positioned by the neurosurgeon to 2 mm above the surface of the root (no direct contact with the tissue) and within 1 mm of the site of ES that elicited the response seen in Fig. 2(a). In Fig. 2(b), EMG recordings taken during INS demonstrate that only the ipsilateral adductor longus muscle (L ADD) is activated through INS of the sensory root. Even in a state of hyperexcitability seen with ES, only one monitored muscle is activated, supporting the spatial precision of INS that has been demonstrated previously (Fig. 2).5 Similar patterns of activation with INS were observed in all seven patients enrolled in this study. In all of the abnormal nerve rootlets included in this study, ES activated four or more muscles on the ipsilateral side, and in some cases, activated additional muscles on the contralateral side. These activation patterns indicate a high degree of spasticity and abnormal innervation. Stimulating the rootlets with INS resulted in activation of three or fewer muscle groups in all responding rootlets with the exception of two adjacent rootlets in one patient. In the one case where this observation did not hold true, all monitored muscles on the ipsilateral side responded to ES and contralateral activation was observed in four and five muscles, respectively. Stimulation of these rootlets with INS resulted in activation of five monitored ipsilateral muscles (only L ADD not activated), but no contralateral muscle activation. This observation indicates that the entire rootlet was not activated by INS, supporting the spatial precision of INS compared to ES in this worst case scenario of spasticity and hyperexcitability. Overall, the electrophysiology results from this study demonstrate the spatial precision of INS relative to ES, in that fewer muscle groups were activated by INS compared to ES for all responding dorsal rootlets in this study. Another feature that differentiates ES from INS is the stimulation artifact seen in the EMG recordings. The ES artifact in Fig. 2(a) obscures the physiologically relevant signal in the EMG trace as the recording amplifier is saturated resulting in the loss of physiologically relevant information. INS evokes potentials on EMG recordings where the distinct evoked potentials are locked to the laser repetition rate (2 Hz) [note arrows repeat at 500 ms in Fig. 2(b)]. No stimulation artifact is present to obscure the evoked potential. The lack of stimulation artifact associated with INS highlights the clinical potential of the technique for neural monitoring applications, where electrical artifacts can obscure subtle evoked neural responses due to the close proximity of neural stimulation and recording sites. 3.2.Infrared Neural Stimulation Can Be Applied Without Damaging Neural TissueA primary goal of this study is to demonstrate safety so that INS can be applied to therapeutic and diagnostic clinical procedures. During this feasibility study in humans, we performed extensive histological analysis of specimen exposed to infrared light. Figure 3(a) presents an example of the normal pathophysiology after laser irradiation (). The axons in this image have a wavy appearance and no discoloration can be seen throughout the image. This section indicates that the near-threshold radiant exposures needed to stimulate the dorsal root do not cause acute damage. An example of thermal damage from laser irradiation () is seen in Figs. 3(b) and 3(c). This lesion contains swelling and hyperchromasia of the collagen in the endoneurium, granular degeneration of the myelin, straightening of the axons, vacuolization, and expansion of endoneurial tubes. Fig. 3Histological comparison of safe versus nonsafe of optically stimulated experimental sites in dorsal lumbar nerve roots. (a) Experimental site ( magnification) resulting in the stimulated muscle recordings using for a total of 20 laser pulses (). Numerous axons (arrows) can be seen in the nerve fibers in this image. (b) An overview of the thermal lesion produced within the dorsal root nerve ( magnification) using (). The lesion is generally hyperchromatic and the endoneurial tubes are straightened out in the center. The arrow represents the interface between the thermally damaged tissue and the underlying normal tissue. (c) The demarcated area from (b) at magnification. This lesion has the following features characteristic of thermal damage: swelling and hyperchromasia of the collagen in the endoneurium (W), granular degeneration of myelin (X), straightening of the nerve fibers as compared to the intact fibers at the bottom of the image, vacuolization, and expansion of the endoneurial tubes in some areas (X). Although some axons show axonal thermal damage (Y), others in the lesion (Z) do not at this level of magnification. 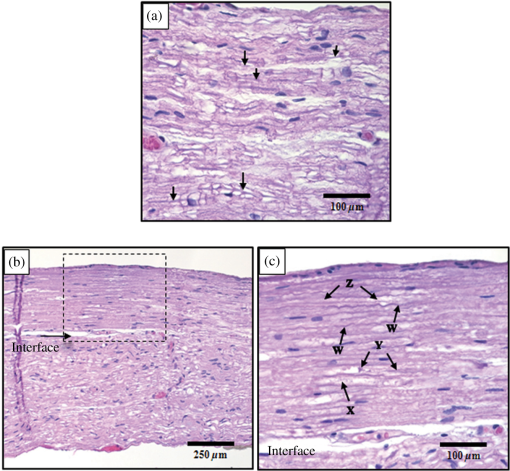 In order to quantify the safety of INS in humans, a three-point grading scale, designed by the blinded neuropathologist (S.T.), was used to grade damage in each sectioned dorsal root. A total of 102 stimulation sites from 35 spinal roots were evaluated using the described damage scoring criteria. Figure 4 displays the results of the histology and the radiant exposures, which resulted in measurable EMG signals. Recordings were obtained from 95 stimulation sites, which resulted in 51 (53%) observed stimulation events. Reliable stimulation was observed for radiant exposures ranging from 0.53 to with an efficacy of 63% (Fig. 4). No measurable response was observed for radiant exposures of 0.503 and , indicating that stimulation threshold of the human dorsal root occurs between 0.50 and . Radiant exposures up to resulted in no damage; however, ambiguous grade 1 damage was first noted at and grade 2 damage first occurred at . The cumulative distribution function generated by Probit analysis of the binary histological data identified the 50% probability of damage to be with 10% probability of damage corresponding to the first observed indication of damage at . The transitional zone (1.00 to ) from nondamaging to damaging radiant exposures using short-term stimulation correlates with the results from animal survival studies, where the 50% probability of damage was 0.91 to .3 The safety ratio, defined as the ratio of radiant exposures where damage is first observed to that where stimulation is first seen, is , the same safety ratio reported in animal studies.3 A slight movement of the surgeon’s hand combined with the physiological motion of the patient during stimulation led to variations in reported radiant exposures due to the divergent beam from the handheld optical fiber probe. Due to this variance in delivered radiant exposures of the tissue target site, the safe stimulation radiant exposure range for human dorsal roots was identified as radiant exposures between 0.53 and because no damage was observed for these reported irradiation levels. The reported efficacy is expected to increase with a collimated beam or fixed optical fiber probe position above the nerve since variance in spot size would be minimized. Fig. 4Identification of safe radiant exposures for stimulation human dorsal roots. Results from the safety and efficacy study from seven patients (102 stimulation sites) graded on a three-point scale. Laser radiant exposure used for each stimulation (20 pulses at 2 Hz recorded for 10 s) is graphed as a function of the thermal damage score assigned to each site based on histopathology from optical stimulation over a range of laser energies (0.46 to ). Probability of damage is given by cumulative distribution function from statistical analysis of binary damage data. Successful stimulations are indicated by blue diamonds, no stimulation is indicated by red dots, and stimulation sites with no EMG recordings are indicated by black triangles. Compound muscle action potentials are first observed at . Stimulation range is identified as 0.53 to . Safe stimulation range is identified as 0.53 to . 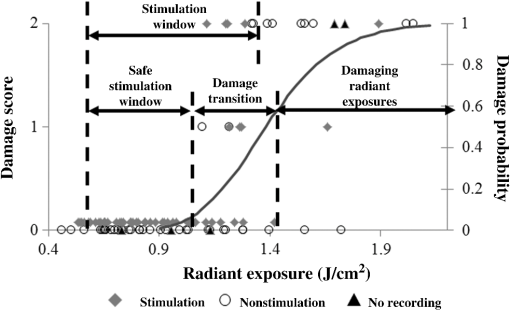 4.DiscussionThis is the first report that INS evokes discrete, precise stimulation of nerve roots in vivo in humans. The advantages of INS, noncontact delivery, artifact-free recordings, and high spatial selectivity were maintained in this study as the neurosurgeon used a handheld probe to stimulate the dorsal root without contacting neural tissue. Single muscle activation was observed during INS within diseased hyperactive dorsal roots, demonstrating the high spatial precision of INS. Histology results explicitly show that we can safely stimulate human nerves without causing acute thermal damage when the intensity of INS is held . The results of this study establish the feasibility needed to support further clinical evaluation of INS in humans. In addition to establishing feasibility of INS in humans, this study documents the first use of INS for sensory root stimulation. Encoding somatosensory information through neural stimulation is an important objective for the neuroprosthetic community in the pursuit of closed looped neural prosthetics.40–42 Activation of efferent neurons in peripheral nerves with INS has been well established in peripheral nerves;2,5,15,16 however, direct evidence of INS-evoked action potentials in peripheral sensory afferents has not been confirmed. The results from this study demonstrate that pulsed infrared light can be used to evoke action potentials in isolated sensory nerves. INS directly evoked an action potential in sensory axons located in dorsal roots that propagated transsynaptically in the spinal cord to activate alpha motor neurons in the sciatic nerve. These results imply that INS may be an appropriate stimulation modality for encoding sensory information in future neural prosthetics with further development. INS of human dorsal spinal roots demonstrates the applicability of this technique for diagnostic use in neurosurgical procedures requiring precise mapping of neural structures. An important diagnostic need in neural monitoring is identification of small groups of neural fibers during surgery where inadvertent injury may result in significant neurological deficits. Examples include neural monitoring during surgery involving peripheral nerve reconstruction, the spinal cord, the central pontine angle, where cranial nerves need to be identified and preserved, or the cavernous nerve surrounding the prostate gland that may be masked by diseased tissue within the region of resection. Each of these examples highlights the concern for inadvertent injury to even a millimeter of neural tissue that may result in significant neurological deficits. This diagnostic need is directly addressed by the high spatial precision of INS. Peripheral nerve monitoring with INS has been well characterized in animal models in mapping sciatic,5 facial,15 and cavernous nerves16. Both the rat sciatic and facial nerves have been mapped with INS to identify regions on the nerve that innervate individual muscles downstream from the stimulation site.5,15 Recently, researchers have demonstrated the ability of infrared light to stimulate subsurface through connective tissue overlaying the rat cavernous nerve evoking a physiological response.16 These studies represent the first steps in developing INS for clinical neural monitoring; however, clinical feasibility in humans had not been directly established until this study. This study is the first report whereby INS was used in human surgery and directly compared with standard ES techniques during spinal root surgery (SDR) establishing the efficacy needed to further investigate clinical neural monitoring with INS in specific applications. The results of this study provide the needed feasibility to initiate clinical trials on the potential value of INS as a therapeutic tool. Researchers have demonstrated that INS safely activates the auditory ganglion cells in the cochlea with better spatial precision and improved auditory frequency encoding when compared to current electrical techniques,21,22,43 and application of INS for stimulating the crista ampullaris identifies the potential for future optical based vestibular implants.20 Recently, pulsed infrared light was shown to pace cardiac tissue in both in vivo and in vitro preparations, establishing the feasibility of optical based pacemakers.23–25 Several studies have demonstrated the ability of pulsed infrared light to modulate neural activity in cortical neurons, highlighting the clinical applicability for central nervous system applications, such as deep brain stimulation and cortical neural prosthetics for brain machine interfaces.7,26–30,44 These example applications of INS given here represent only a small percentage of the possible applications where INS may improve the standard clinical care. The high spatial selectivity of INS will allow clinicians to stimulate subfascicular for peripheral nerve reconstruction, such as brachial nerve plexus or otolaryngology applications, where surgeons have a need to identify small groups of fibers to determine function and connectivity.45,46 Possible functional applications of INS include incorporation of the stimulation modality in neural prosthesis used to restore function, block pain, or treat movement disorders. Functional ES has been used to provide control of the bladder, reanimate paralyzed limbs, control pain, and treat movement disorders.47–50 In most of these applications, the patient may experience adverse side effects to the functional ES attributed to current spread. The lack of current spread associated with INS makes it a viable alternative to ES in these applications. While this pilot study demonstrates that INS can stimulate human nerves safely, inherent limitations in the study design may have adversely affected the results of the study, requiring further discussion. First, the neural tissue stimulated in this study was hyperactive dorsal roots caused by cerebral palsy, as these abnormal dorsal roots may be more or less responsive than normal neural tissue or other pathological conditions. This study limitation was unavoidable as ethical considerations required neural tissue that was sacrificed as part of the standard procedure. Additionally, the sacrificed dorsal roots allowed the initial safety of INS in humans to be established. The overall impact of this study weakness is limited as the expected safety ratio, previously demonstrated in animal experiments, was observed,3 and a significant effect from the hyperactivity or diseased state of the dorsal roots would have altered the expected safety ratio. Further, other studies investigating INS have demonstrated that the technique must be optimized for each new application due to different tissue geometries and function.5–7,15,17,22,25,30 Optimization of INS for human applications will be required, but as this study demonstrates, preclinical results will likely translate to clinical application, indicating most optimization can be achieved in preclinical models. The handheld probe utilized during the course of this study was determined to be a limitation due to uncontrolled variability in radiant exposures caused by difficulty in maintaining the probe to tissue distance at 1.5 mm. Small changes in the probe tip to tissue distance significantly changes the radiant exposure delivered to the tissue due to the divergent beam from the probe. If the distance to tissue is , then the radiant exposure increases, while it decreases if the distance is . Based on the results from this study, the variability in probe to tissue distance was likely skewed toward distances . Stimulation and damage thresholds were slightly elevated when compared to preclinical data in the rat sciatic nerve,3 and no damage was observed in the specimen exposed up to , a clear damaging radiant exposure. These observations support the assertion that error related to probe to tissue distance was skewed toward distances , resulting in reduced radiant exposures. Further, stimulation efficacy was only 63% over the effective stimulation range, and decreasing the variability in probe to tissue distance is predicted to increase the overall efficacy of INS in this application. The variability associated with spot size can be minimized by a fixed-point source or a collimated beam. A fixed-point source places the source at a specified distance from the tissue standardizing the spot size of the beam. This concept has been demonstrated in most preclinical INS studies;5,15,25,29 however, this form of light delivery is most applicable in implants where the light source is designed into a medical device22 and may not be advantageous in surgical applications where contact-free stimulation is desired. A collimated beam incorporated into a handheld probe would allow for contact-free stimulation in diagnostic neurosurgical applications. The collimated beam is produced by incorporating advanced optics (i.e., gradient-indexed lens) into the handheld probe to create a beam with a standardized width over a large working distance (i.e., ). This concept has previously been demonstrated by Fried and colleagues who demonstrated that a collimated beam reduced the stimulation threshold and increased the reliability of INS when compared to a standard Gaussian divergent beam.17,51 Our immediate future research efforts are focused on optimization of a handheld collimated probe designed for diagnostic neural monitoring and characterizing the effects collimation has on stimulation and damage threshold in preclinical models before continuing INS clinical studies. We believe a change to a collimated handheld probe will eliminate the variability associated with radiant exposures observed in this study, improving the overall efficacy and safety of the technique in humans. 5.ConclusionsAs more researchers enter the INS field and new devices are developed, the number of applications for this technique will continue to grow; however, the clinical potential of INS could not be realized until efficacy and safety was demonstrated in humans. In this study, pulsed infrared light was found to evoke neural activity in human dorsal spinal roots only activating one or two muscle groups demonstrating high spatial precision, and INS was found to have a safety ratio establishing a safe range of radiant exposures that could be used to stimulate human neural tissue. This study provides the first successful steps needed to translate this new optical technology to the clinic and encourages the scientific community to rethink the accepted paradigm of neural activation to beyond electrical methods. AcknowledgmentsThe work was supported by the W. M. Keck Foundation Free Electron Laser Center, MFEL/AFOSR program Grant Number FA9550-04-1-0045 and by the National Institutes of Health Grant Number R01 NS052407-01. The authors would like to acknowledge Melba Isom for assisting in consenting of patients and maintaining the study with the Vanderbilt IRB. We also thank John Scarafiotti and Sentient Medical Systems (SMS, Cockeysville, Maryland), a contracted surgical monitoring service that provided routine intraoperative monitoring (IOM) for these rhizotomy cases, for use of existing operating room equipment, and IOM personnel and the surgical nursing staff of Vanderbilt Children’s Hospital. ReferencesJ. Wells et al.,
“Optical stimulation of neural tissue in vivo,”
Opt. Lett., 30
(5), 504
–506
(2005). http://dx.doi.org/10.1364/OL.30.000504 OPLEDP 0146-9592 Google Scholar
J. Wells et al.,
“Application of infrared light for in vivo neural stimulation,”
J. Biomed. Opt., 10
(6), 064003
(2005). http://dx.doi.org/10.1117/1.2121772 JBOPFO 1083-3668 Google Scholar
J. Wells et al.,
“Optically mediated nerve stimulation: identification of injury thresholds,”
Lasers Surg. Med., 39
(6), 513
–526
(2007). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
J. Wells et al.,
“Biophysical mechanisms of transient optical stimulation of peripheral nerve,”
Biophys. J., 93
(7), 2567
–2580
(2007). http://dx.doi.org/10.1529/biophysj.107.104786 BIOJAU 0006-3495 Google Scholar
J. Wells et al.,
“Pulsed laser versus electrical energy for peripheral nerve stimulation,”
J. Neurosci. Methods, 163
(2), 326
–337
(2007). http://dx.doi.org/10.1016/j.jneumeth.2007.03.016 JNMEDT 0165-0270 Google Scholar
A. R. Duke et al.,
“Spatial and temporal variability in response to hybrid electro-optical stimulation,”
J. Neural Eng., 9
(3), 036003
(2012). http://dx.doi.org/10.1088/1741-2560/9/3/036003 1741-2560 Google Scholar
J. M. Cayce et al.,
“Infrared neural stimulation of primary visual cortex in non-human primates,”
Neuroimage, 84 181
–190
(2014). http://dx.doi.org/10.1016/j.neuroimage.2013.08.040 NEIMEF 1053-8119 Google Scholar
G. Tommasi et al.,
“Pyramidal tract side effects induced by deep brain stimulation of the subthalamic nucleus,”
J. Neurol., Neurosurg. Psychiatry, 79
(7), 813
–819
(2008). http://dx.doi.org/10.1136/jnnp.2007.117507 JNNPAU 0022-3050 Google Scholar
D. M. Landsberger and A. G. Srinivasan,
“Virtual channel discrimination is improved by current focusing in cochlear implant recipients,”
Hearing Res., 254
(1–2), 34
–41
(2009). http://dx.doi.org/10.1016/j.heares.2009.04.007 HERED3 0378-5955 Google Scholar
J. P. Donoghue, S. Leibovic and J. N. Sanes,
“Organization of the forelimb area in squirrel monkey motor cortex: representation of digit, wrist, and elbow muscles,”
Exp. Brain Res., 89
(1), 1
–19
(1992). http://dx.doi.org/10.1007/BF00228996 EXBRAP 0014-4819 Google Scholar
D. Popovic et al.,
“Properties of implanted electrodes for functional electrical stimulation,”
Ann. Biomed. Eng., 19
(3), 303
–316
(1991). http://dx.doi.org/10.1007/BF02584305 ABMECF 0090-6964 Google Scholar
C. Strauss,
“The facial nerve in medial acoustic neuromas,”
J. Neurosurg., 97
(5), 1083
–1090
(2002). http://dx.doi.org/10.3171/jns.2002.97.5.1083 JONSAC 0022-3085 Google Scholar
M. L. Kircher and J. M. Kartush,
“Pitfalls in intraoperative nerve monitoring during vestibular schwannoma surgery,”
Neurosurg. Focus, 33
(3), 5
(2012). http://dx.doi.org/10.3171/2012.7.FOCUS12196 1092-0684 Google Scholar
H. Wiedemayer et al.,
“The impact of neurophysiological intraoperative monitoring on surgical decisions: a critical analysis of 423 cases,”
J. Neurosurg., 96
(2), 255
–262
(2002). http://dx.doi.org/10.3171/jns.2002.96.2.0255 JONSAC 0022-3085 Google Scholar
I. U. Teudt et al.,
“Optical stimulation of the facial nerve: a new monitoring technique?,”
Laryngoscope, 117
(9), 1641
–1647
(2007). http://dx.doi.org/10.1097/MLG.0b013e318074ec00 LARYA8 0023-852X Google Scholar
S. Tozburun et al.,
“Subsurface near-infrared laser stimulation of the periprostatic cavernous nerves,”
J. Biophotonics, 5
(10), 793
–800
(2012). http://dx.doi.org/10.1002/jbio.201100134 JBOIBX 1864-063X Google Scholar
S. Tozburun et al.,
“Infrared laser nerve stimulation as a potential diagnostic method for intra-operative identification and preservation of the prostate cavernous nerves,”
IEEE J. Sel. Topics Quantum Electron., 20
(2), 299
–306
(2014). http://dx.doi.org/10.1109/JSTQE.2013.2293273 IJSQEN 1077-260X Google Scholar
E. Peterson and D. Tyler,
“Motor neuron activation in peripheral nerves using infrared neural stimulation,”
J. Neural Eng., 11
(1), 016001
(2014). http://dx.doi.org/10.1088/1741-2560/11/1/016001 1741-2560 Google Scholar
C. P. Richter et al.,
“Spread of cochlear excitation during stimulation with pulsed infrared radiation: inferior colliculus measurements,”
J. Neural Eng., 8
(5), 056006
(2011). http://dx.doi.org/10.1088/1741-2560/8/5/056006 1741-2560 Google Scholar
S. M. Rajguru et al.,
“Infrared photostimulation of the crista ampullaris,”
J. Physiol., 589
(6), 1283
–1294
(2011). http://dx.doi.org/10.1113/jphysiol.2010.198333 JPHYA7 0022-3751 Google Scholar
A. D. Izzo et al.,
“Laser stimulation of the auditory nerve,”
Lasers Surg. Med., 38
(8), 745
–753
(2006). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
A. I. Matic et al.,
“Behavioral and electrophysiological responses evoked by chronic infrared neural stimulation of the cochlea,”
PLoS One, 8
(3), e58189
(2013). http://dx.doi.org/10.1371/journal.pone.0058189 1932-6203 Google Scholar
M. W. Jenkins et al.,
“Optical pacing of the embryonic heart,”
Nat. Photon., 4
(9), 623
–626
(2010). http://dx.doi.org/10.1038/nphoton.2010.166 1749-4885 Google Scholar
G. M. Dittami et al.,
“Intracellular calcium transients evoked by pulsed infrared radiation in neonatal cardiomyocytes,”
J. Physiol., 589
(6), 1295
–1306
(2011). http://dx.doi.org/10.1113/jphysiol.2010.198804 JPHYA7 0022-3751 Google Scholar
M. W. Jenkins et al.,
“Optical pacing of the adult rabbit heart,”
Biomed. Opt. Express, 4
(9), 1626
–1635
(2013). http://dx.doi.org/10.1364/BOE.4.001626 BOEICL 2156-7085 Google Scholar
J. M. Cayce et al.,
“Pulsed infrared light alters neural activity in rat somatosensory cortex in vivo,”
Neuroimage, 57
(1), 155
–166
(2011). http://dx.doi.org/10.1016/j.neuroimage.2011.03.084 NEIMEF 1053-8119 Google Scholar
H.-J. Feng et al.,
“Alteration of GABAergic neurotransmission by pulsed infrared laser stimulation,”
J. Neurosci. Methods, 192
(1), 110
–114
(2010). http://dx.doi.org/10.1016/j.jneumeth.2010.07.014 JNMEDT 0165-0270 Google Scholar
J. M. Cayce et al.,
“Infrared neural stimulation of thalamocortical brain slices,”
IEEE J. Sel. Topics Quantum Electron., 16
(3), 565
–572
(2010). http://dx.doi.org/10.1109/JSTQE.2009.2032424 IJSQEN 1077-260X Google Scholar
J. M. Cayce et al.,
“Calcium imaging of infrared-stimulated activity in rodent brain,”
Cell Calcium, 55
(4), 183
–190
(2014). http://dx.doi.org/10.1016/j.ceca.2014.01.004 CECADV 0143-4160 Google Scholar
M. Yoo et al.,
“Near-infrared stimulation on globus pallidus and subthalamus,”
J. Biomed. Opt., 18
(12), 128005
(2013). http://dx.doi.org/10.1117/1.JBO.18.12.128005 JBOPFO 1083-3668 Google Scholar
A. C. Thompson et al.,
“Modeling of the temporal effects of heating during infrared neural stimulation,”
J. Biomed. Opt., 18
(3), 035004
(2013). http://dx.doi.org/10.1117/1.JBO.18.3.035004 JBOPFO 1083-3668 Google Scholar
M. G. Shapiro et al.,
“Infrared light excites cells by changing their electrical capacitance,”
Nat. Commun., 3 736
(2012). http://dx.doi.org/10.1038/ncomms1742 NCAOBW 2041-1723 Google Scholar
E. S. Albert et al.,
“TRPV4 channels mediate the infrared laser-evoked response in sensory neurons,”
J. Neurophysiol., 107
(12), 3227
–3234
(2012). http://dx.doi.org/10.1152/jn.00424.2011 JONEA4 0022-3077 Google Scholar
K. Salame et al.,
“Surgical treatment of spasticity by selective posterior rhizotomy: 30 years experience,”
Isr Med. Assoc. J., 5
(8), 543
–546
(2003). IMAJCX 1565-1088 Google Scholar
A. L. Albright,
“Neurosurgical treatment of spasticity and other pediatric movement disorders,”
J. Child Neurol., 18
(Suppl. 1), S67
–78
(2003). JOCNEE 0883-0738 Google Scholar
T. Park,
“Selective dorsal rhizotomy for the spasticity of cerebral palsy,”
Neurosurgical Operative Atlas, 183
–190 American Association of Neurological Surgeons, Park Ridge, IL
(1994). Google Scholar
T. S. Park and J. M. Johnston,
“Surgical techniques of selective dorsal rhizotomy for spastic cerebral palsy. Technical note,”
Neurosurg. Focus, 21
(2), e7
(2006). Google Scholar
S. Thomsen,
“Mapping thermal injury in biologic tissues using quantitative pathologic techniques,”
Proc. SPIE, 3594 82
(1999). http://dx.doi.org/10.1117/12.348748 PSISDG 0277-786X Google Scholar
B. J. Lund,
“The probitfit program to analyze data from laser damage threshold studies,”
Brooks City-Base, Texas
(2006). Google Scholar
H. Scherberger,
“Neural control of motor prostheses,”
Curr. Opin. Neurobiol., 19
(6), 629
–633
(2009). http://dx.doi.org/10.1016/j.conb.2009.10.008 COPUEN 0959-4388 Google Scholar
P. D. Marasco, A. E. Schultz and T. A. Kuiken,
“Sensory capacity of reinnervated skin after redirection of amputated upper limb nerves to the chest,”
Brain, 132
(6), 1441
–1448
(2009). http://dx.doi.org/10.1093/brain/awp082 BRAIAK 0006-8950 Google Scholar
D. J. Weber et al.,
“Limb-state information encoded by peripheral and central somatosensory neurons: implications for an afferent interface,”
IEEE Trans. Neural Syst. Rehabil. Eng., 19
(5), 501
–513
(2011). http://dx.doi.org/10.1109/TNSRE.2011.2163145 ITNSB3 1534-4320 Google Scholar
S. M. Rajguru et al.,
“Optical cochlear implants: evaluation of surgical approach and laser parameters in cats,”
Hearing Res., 269
(1–2), 102
–111
(2010). http://dx.doi.org/10.1016/j.heares.2010.06.021 HERED3 0378-5955 Google Scholar
X. Y. Wu et al.,
“Irradiation of 850-nm laser light changes the neural activities in rat primary visual cortex,”
Lasers Med. Sci., 28
(3), 791
–798
(2013). http://dx.doi.org/10.1007/s10103-012-1160-x LMSCEZ 1435-604X Google Scholar
H. C. Martin et al.,
“Patient-assessed outcomes after excision of acoustic neuroma: postoperative symptoms and quality of life,”
J. Neurosurg., 94
(2), 211
–216
(2001). http://dx.doi.org/10.3171/jns.2001.94.2.0211 JONSAC 0022-3085 Google Scholar
H. Ueno et al.,
“Endoscopic carpal tunnel release and nerve conduction studies,”
Int. Orthop., 24
(6), 361
–363
(2001). http://dx.doi.org/10.1007/s002640000189 0341-2695 Google Scholar
T. Changfeng et al.,
“Bladder inhibition or voiding induced by pudendal nerve stimulation in chronic spinal cord injured cats,”
Neurourol. Urodyn., 26
(4), 570
–577
(2007). http://dx.doi.org/10.1002/(ISSN)1520-6777 NEUREM 0733-2467 Google Scholar
V. Gritsenko and A. Prochazka,
“A functional electric stimulation—assisted exercise therapy system for hemiplegic hand function,”
Arch. Phys. Med. Rehabil., 85
(6), 881
–885
(2004). http://dx.doi.org/10.1016/j.apmr.2003.08.094 APMHAI 0003-9993 Google Scholar
F. Fregni et al.,
“A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury,”
Pain, 122
(1–2), 197
–209
(2006). http://dx.doi.org/10.1016/j.pain.2006.02.023 PAINDB 0304-3959 Google Scholar
P. Konrad and T. Shanks,
“Implantable brain computer interface: challenges to neurotechnology translation,”
Neurobiol. Dis., 38
(3), 369
–375
(2010). http://dx.doi.org/10.1016/j.nbd.2009.12.007 NUDIEM 0969-9961 Google Scholar
N. M. Fried et al.,
“Noncontact stimulation of the cavernous nerves in the rat prostate using a tunable-wavelength thulium fiber laser,”
J. Endourol., 22
(3), 409
–414
(2008). http://dx.doi.org/10.1089/end.2008.9996 JENDE3 0892-7790 Google Scholar
BiographyJonathan M. Cayce received his BS degree in biomedical engineering from the University of Alabama at Birmingham in 2006. He received his MS degree and his PhD degree in 2013 from Vanderbilt University, both in biomedical engineering. He is currently a clinical research scientist at DeRoyal Industries. Jonathon D. Wells received his BS, MS, and PhD in the field of biomedical engineering from Vanderbilt University. He is currently a program manager at Lockheed Martin. Jonathan D. Malphrus received his BS degree in ceramic and material engineering from Clemson University in 2006. He is currently owner of Steric Designs, a custom furniture design and fabrication company. Chris Kao received his MD degree from Bethune Medical College, Changchun, China, in 1983 and his PhD degree in neurophysiology from the Medical College of Virginia in 1994. He is currently a research associate professor of neurological surgery at Vanderbilt University and the director of Sentient Medical Systems–Vanderbilt Deep Brain Stimulation Program. His clinical interest includes microelectrode brain mapping for target localization of deep brain stimulation treatment of movement disorders. Sharon Thomsen received her AB degree from the University of Washington and her MD degree from Stanford University, Stanford, CA. She is currently a retired pathologist who continues to consult with engineers and physicists. Her research interests have centered upon using qualitative and quantitative pathologic techniques to investigate the interactions of nonionizing electromagnetic radiation on biologic tissues with emphasis on light transport and thermal effects. Noel B. Tulipan received his MD degree from Johns Hopkins University, Baltimore, Maryland. He is currently a professor of neurological surgery at Vanderbilt University, and he specializes in all aspects of pediatric neurosurgery with special emphasis on congenital defects of the nervous system, hydrocephalus, craniofacial reconstruction, and surgery for spasticity. Peter E. Konrad received his BA degree in biology and chemistry from Rockford College. He received his MS degree in cardiovascular bioengineering and physiology, his PhD degree in neurophysiology from Purdue University, and his MD degree from Indiana University. He is currently professor of neurosurgery and biomedical engineering at Vanderbilt University. His clinical interest is in all areas of functional neurosurgery, with special emphasis on the surgical treatment of movement disorders. E. Duco Jansen received his M.S. (Drs.) degree in medical sciences from the University of Utrecht, and his MS and PhD degrees in biomedical engineering from the University of Texas. He is currently professor of biomedical engineering and neurosurgery at Vanderbilt University and is the associate dean for graduate studies in the School of Engineering. His research focus is on application of light, lasers, and optical technology in medicine and biology. Anita Mahadevan-Jansen received her BS and MS degrees in physics from the University of Bombay and her MS and PhD degrees in biomedical engineering from the University of Texas. She is the Orrin H. Ingram professor of biomedical engineering and professor of neurological surgery at Vanderbilt University. Her research focus is on application of optical techniques for diagnosis of pathology, stimulation of neural tissue, and translation of optical technology for clinical applications. |

